Background
Microbubble Ultrasound Contrast Agents
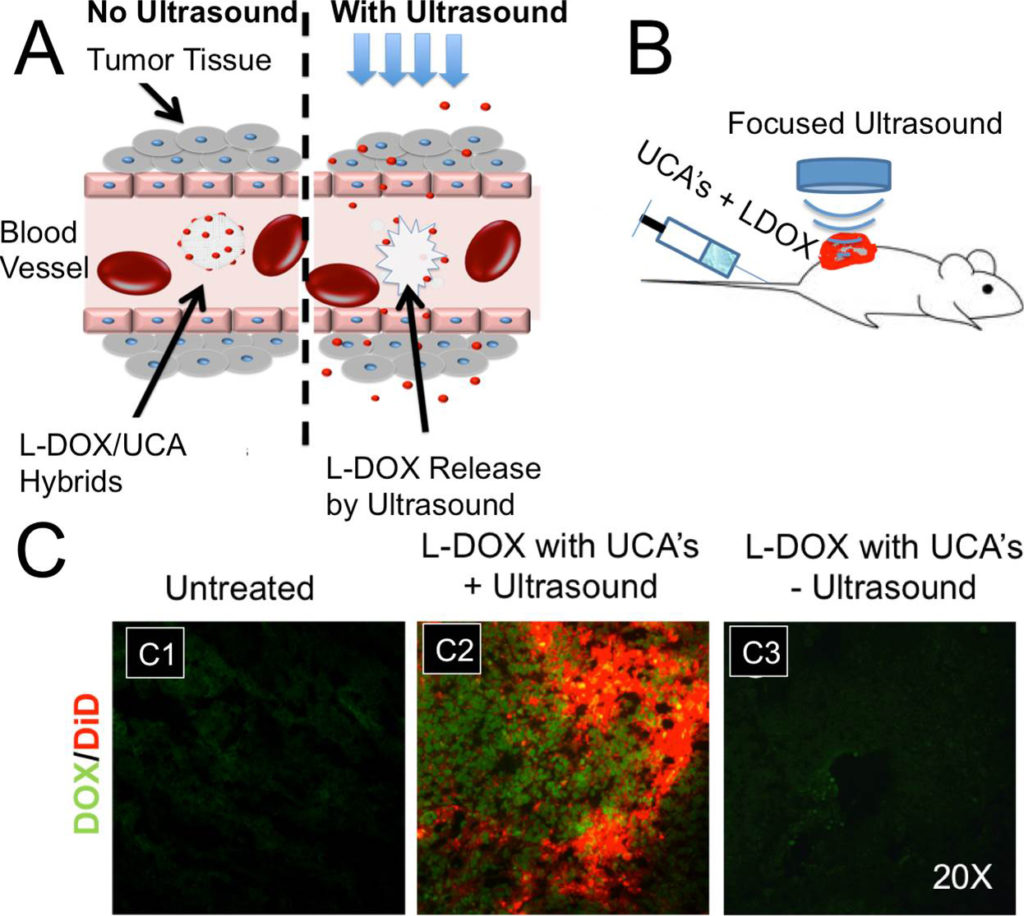
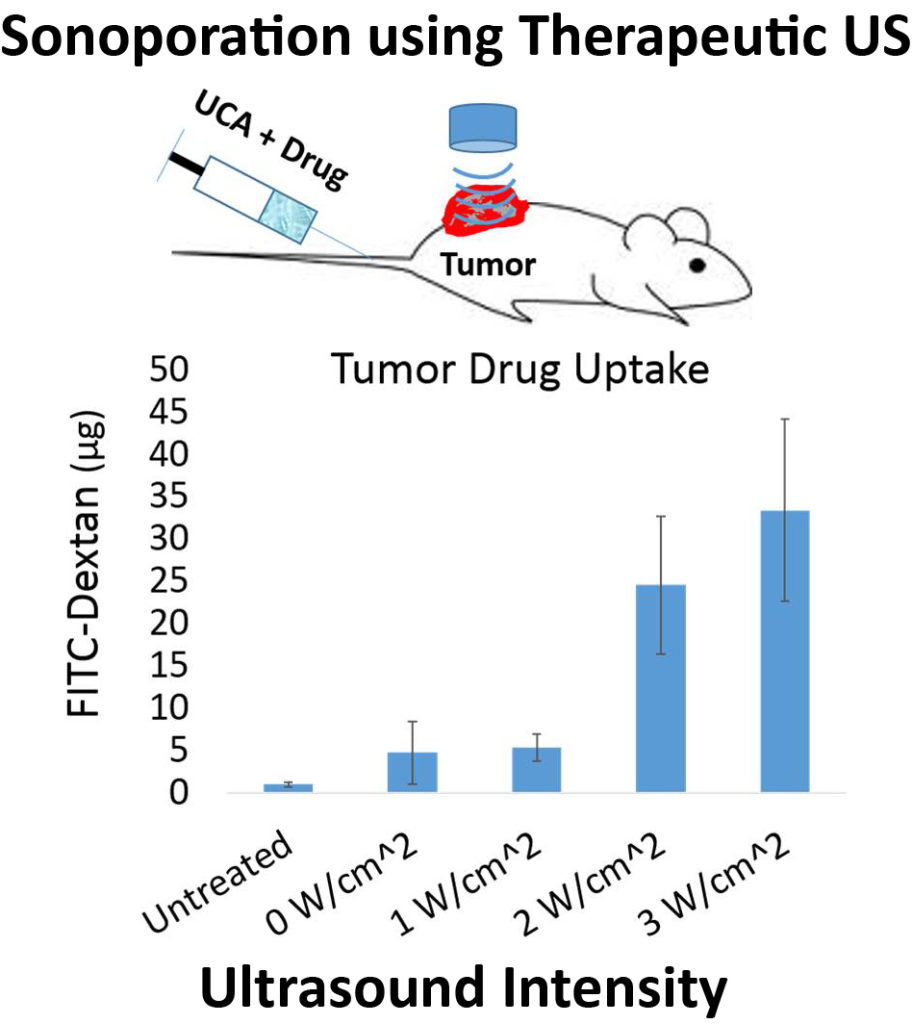
Ultrasound contrast agents (UCA’s) are currently being developed for a wide variety of biomedical applications beyond their intended purpose. UCA’s are multifunctional vascular probes (between 1-10 µm in diameter) comprised of a thin shell and gas core. The compressible gas core of the microbubble allows them to scatter sound waves more effectively than surrounding blood and tissue, making them detectable in the body with clinical ultrasound scanners. Due to their inherent size, microbubbles remain in the blood when injected systemically, thus making them excellent vascular probes. The most common application of UCA’s is for monitoring blood perfusion , but UCA’s are also being developed for targeted drug delivery applications in vivo. At high amplitudes of ultrasound pressure, the mechanical response of the microbubbles to the ultrasound wave can permeabilize the vascular endothelial layer and facilitate therapeutic drug extravasation only at the site of ultrasound application.
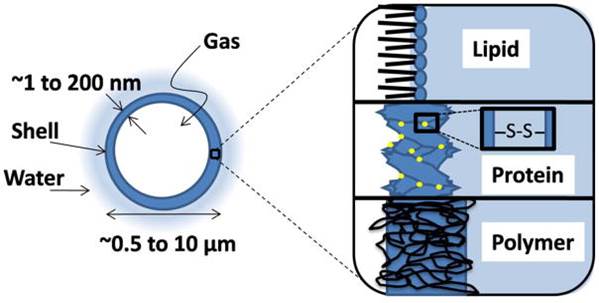
Image-Guided Drug Delivery
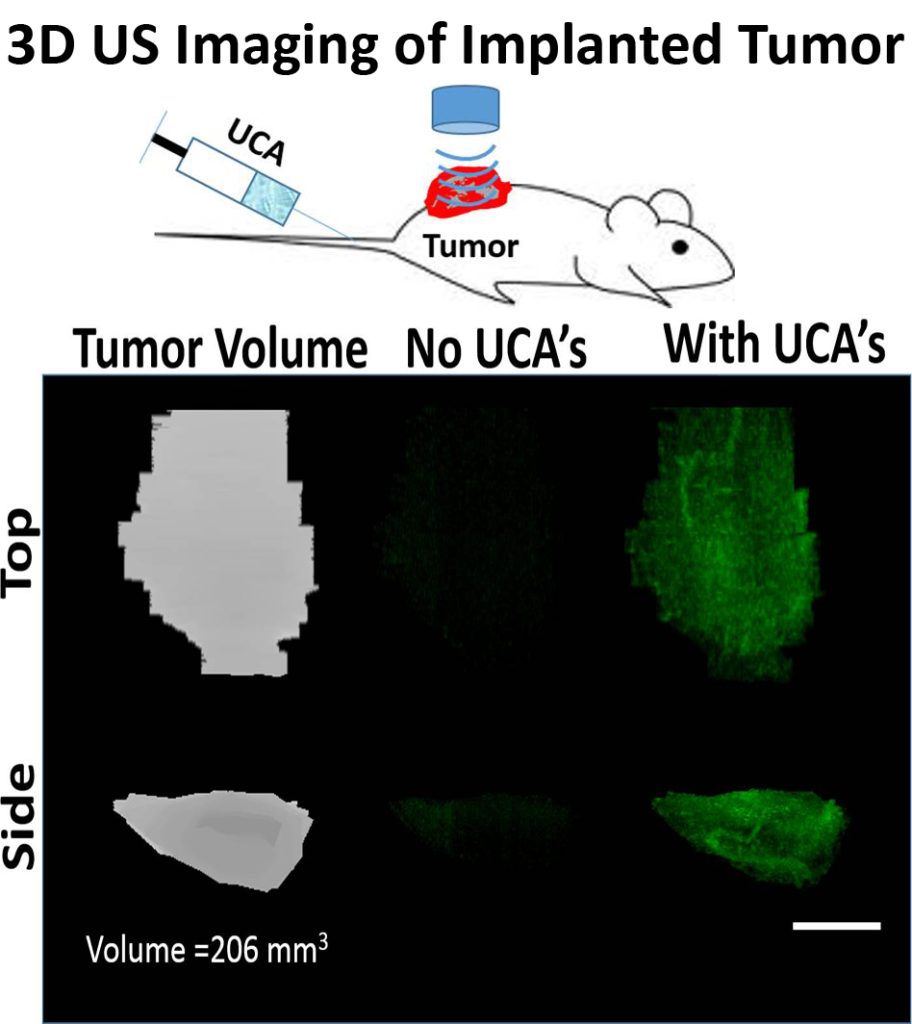
We are currently working on developing in vivo imaging techniques for monitoring tumor drug permeability utilizing UCA’s. Currently, no clinical methods exist to simultaneously monitor and optimize drug uptake in vivo. Our group is currently exploring the immediate effects of sonoporation on tumor perfusion kinetics and the morphology of the tumor microvasculature (NIH-1R21EB015040-01A1). We seek to build on our recent progress and explore the ability to utilize UCA perfusion kinetics to optimize drug uptake during combinatorial treatment in both pediatric tumor models tumors. We hypothesize that UCA perfusion dynamics can be used to evaluate response to therapy and identify time-points when tumors are most amenable to drug uptake, and optimize delivery of chemotherapeutics over the course of treatment using sonoporation techniques. The techniques developed in this study would be particularly relevant in a clinical setting for longitudinal drug therapies where the tumor vasculature is dynamically changing in response to treatment.
3D Perfusion Imaging
Focused Ultrasound Mediated Drug Delivery
Novel Ultrasound Contrast Agents
Novel UCA’s for Improving Drug Delivery
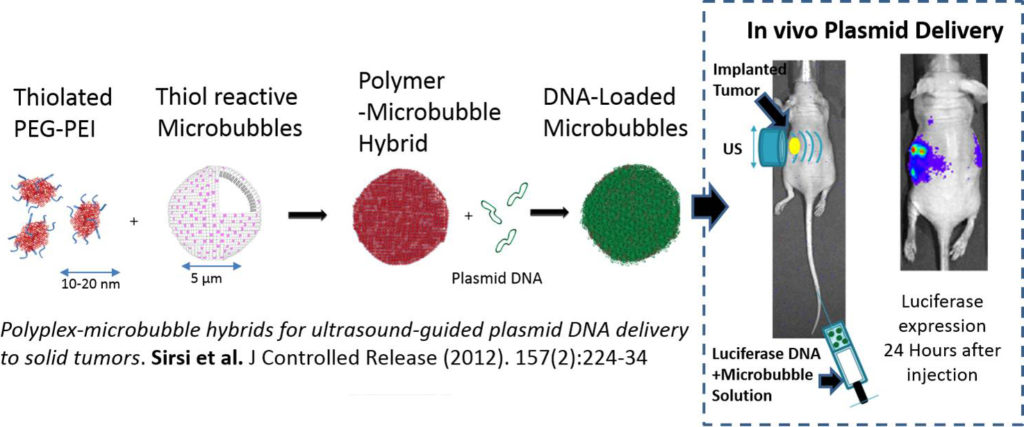
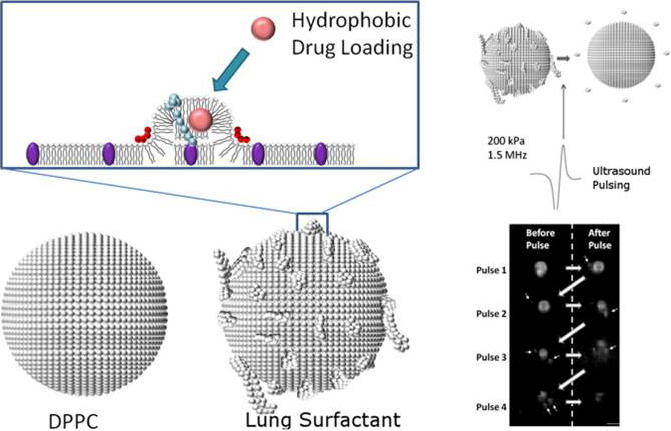
We develop novel UCA’s to improve image-guided drug therapies. We have previously developed novel size-selected polymer-lipid hybrid UCA’s that demonstrated increased nucleic acid loading capacity and improved site-specific gene delivery. We have also developed “bulky” high surface area lipid bubbles for improved loading of lipophilic drugs. We plan to continue developing novel UCA’s to optimize drug and nucleic acid delivery in targeted drug/gene delivery applications. Drug loaded UCA’s have the potential to treat numerous cancers, as well as a wide variety of other genetic disorders, using therapeutic plasmids and siRNA’s.