The Jones Lab is a multidisciplinary research team focused on bridging cutting edge engineering disciplines with immunology to prevent, diagnose, and treat immune-related disorders. We focus our studies on: (1) Engineering innovative lab-on-a-chip platforms to model and study immune cell-pathogen interactions with single cell precision; (2) Developing imaging techniques and implantable sensors to track and monitor real-time host-pathogen interactions (e.g. biomarkers, cells) in animal models; (3) Merging quantitative single-cell measurements and mathematical modeling with the goal of understanding how a patient’s unique immune signature correlates to clinical outcome; (4) Elucidating the effects of microenvironment on cancer cell spheroid growth and tumor-immune cell crosstalk, on-chip.
MICROFLUIDICS TO QUANTIFY HOST-PATHOGEN INTERACTIONS
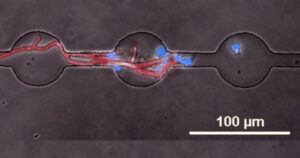
The dynamic interactions between host and pathogen are essential in determining the extent of immune response and consequent infection outcome. On the single cell level, cell-to-cell variability and random molecular movements among bacterial pathogens present a variety of stochastic encounters for host immune cells. These scenarios generate a diverse set of immune cell phenotypes often masked by conventional bulk measurements. Microfluidic devices provide a high-throughput platform for single-cell host pathogen studies. The device microenvironment can be precisely tuned, permitting the isolation and quantification of immune cell phenotypes in a variety of clinically relevant conditions. Identifying novel minority phenotypes among the immunocyte majority can lead to more accurate diagnoses and predictions of clinical outcome.
BIOSENSORS FOR SINGLE CELL ANALYSIS
A biosensor is an analytical device that incorporates a biological component with a physicochemical detector in order to detect the presence and quantity of an analyte. The Jones Lab is working to develop biosensors that are able to quantify immunologically relevant cytokines being secreted from neutrophils and macrophages at a single cell level. The wide heterogeneity in the quantity and type of cytokines released from an individual cell type during a condition such as Sepsis, makes the single cell model highly valuable. Additionally, our biosensors enable us to monitor how the amount of cytokine being released from a cell varies over time.
MATHEMATICAL MODELING FOR IMMUNE CELL DYNAMICS DURING SEPSIS
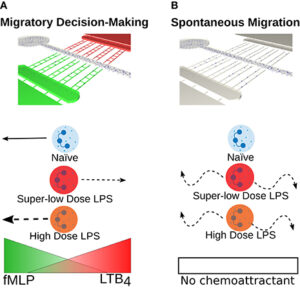
Our experiments are performed on a single cell level with high temporal resolution, thus we generate massive amounts of data on immune cell phenotypes. A method to analyze and utilize this data is to create mathematical models. Cellular behavior seen experimentally can be translated and captured in a set of equations. Mathematical models allow us to effectively interpret experimental data and form both qualitative and quantitative conclusions on our data. Complex molecular interactions within cell processes can also be illustrated via mathematical models. This aids us in understand the underlying mechanisms of immune cell phenotypes. These models can also be used to create hypotheses that can influence future experiments. This multidisciplinary approach to questions in immunology can not only recapitulate known biological processes but help further our understanding by formulating new testable hypotheses.
THE MICROENVIRONMENT OF CANCER AND METASTASIS
Circulating Tumor Cells (CTCs) are found in the peripheral blood of patients with solid tumors and have been isolated successfully but in low numbers. Keeping the cells viable for analysis has been a technical challenge and this has become a hindrance to using CTCs reliably in diagnostic tests. The advent of microfluidics in biology has been a major driving force for application of microsystems in medical diagnostics and lab-on-a-chip technologies. The Jones Lab specializes in designing and fabricating novel and highly integrated microfluidic devices for CTCs and their application in cancer diagnostics.