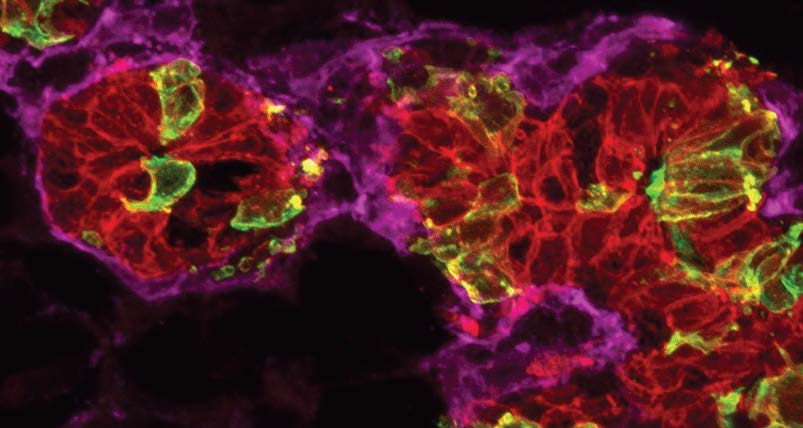
In the mammary gland, we have found that stem cells are significantly responsive to ovarian hormones and increase in numbers during the female reproductive cycle to achieve epithelial tissue expansion in milk-producing alveolar sacs in anticipation of pregnancy. We uncovered cellular cross-talk mechanisms and signaling pathways in the stem cell niche that are central for stem cell and progenitor cell behavior. The same hormones and signaling mechanisms that drive stem cells are known to increase the risk of breast cancer, and thus our research provides insight on how stem cells in the mammary gland are likely recruited to drive breast cancer pathogenesis. Further, we have identified a novel PDGFRα+ PREF-1+ adipogenic mesenchymal progenitor in the mammary stroma that generates epithelial cell types in the mammary parenchyma during development and hormone-driven adult tissue expansion. These mesenchymal cells are present in other mammalian tissues and we are interested in advancing our understanding of their biological significance and molecular mechanisms during tissue regeneration and cancer.
Our research projects include:
- The role of mesenchymal progenitors during mammary development and regeneration:
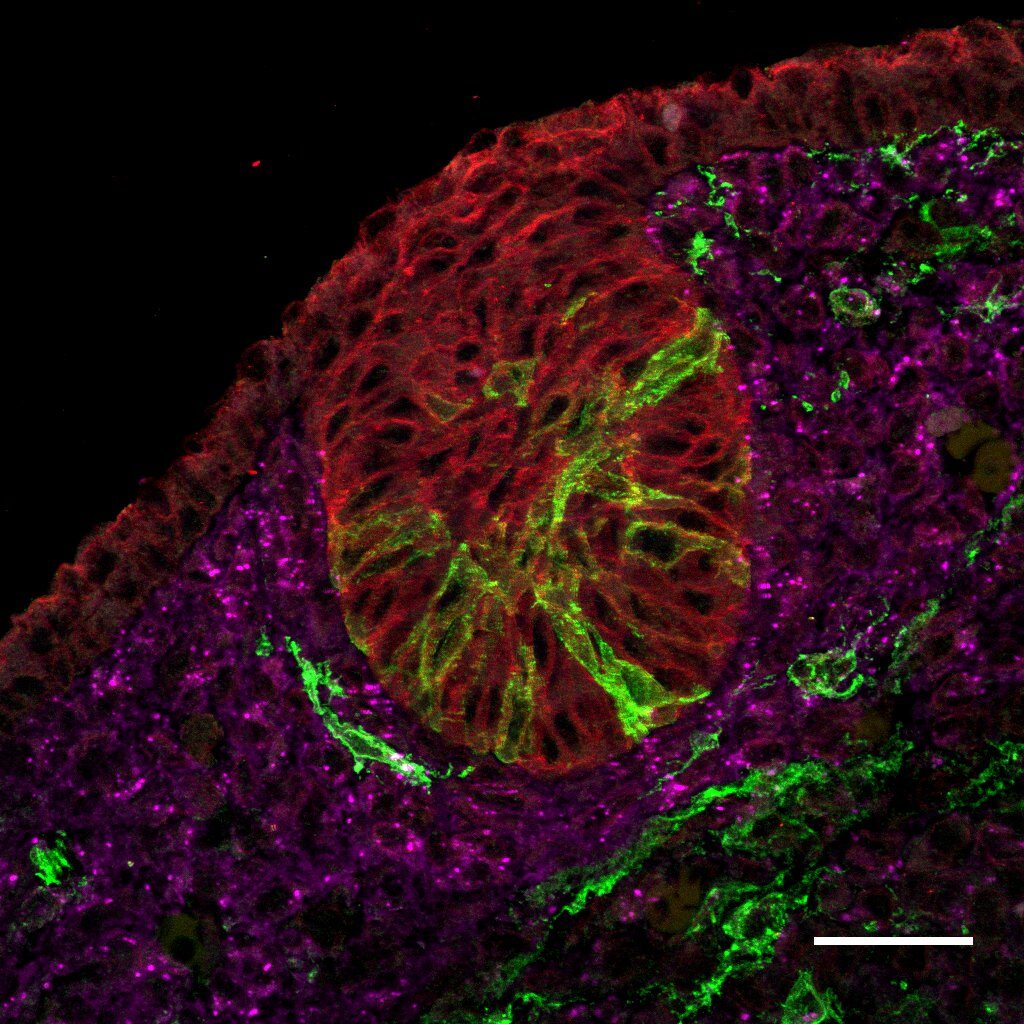
PDGFRα marks mesenchymal progenitors in the mammary microenvironment that can transition to epithelial cells, but its precise role during development and regeneration is unknown. We are studying the role of PDGFRα both in vivo and in vitro using biochemical and genetic approaches. We are employing lineage tracing mouse models and cell ablation/knockout systems to investigate the functional requirement of mesenchymal progenitors in early mammary development, adult tissue homeostasis and regenerative phases. We are interested in identifying PDGFRα signaling targets in these unique cells during distinct windows of tissue growth and interrogating their impact in the microenvironment.
- The fate of PDGFRα+ progenitors in adiposity, cancer development and progression:
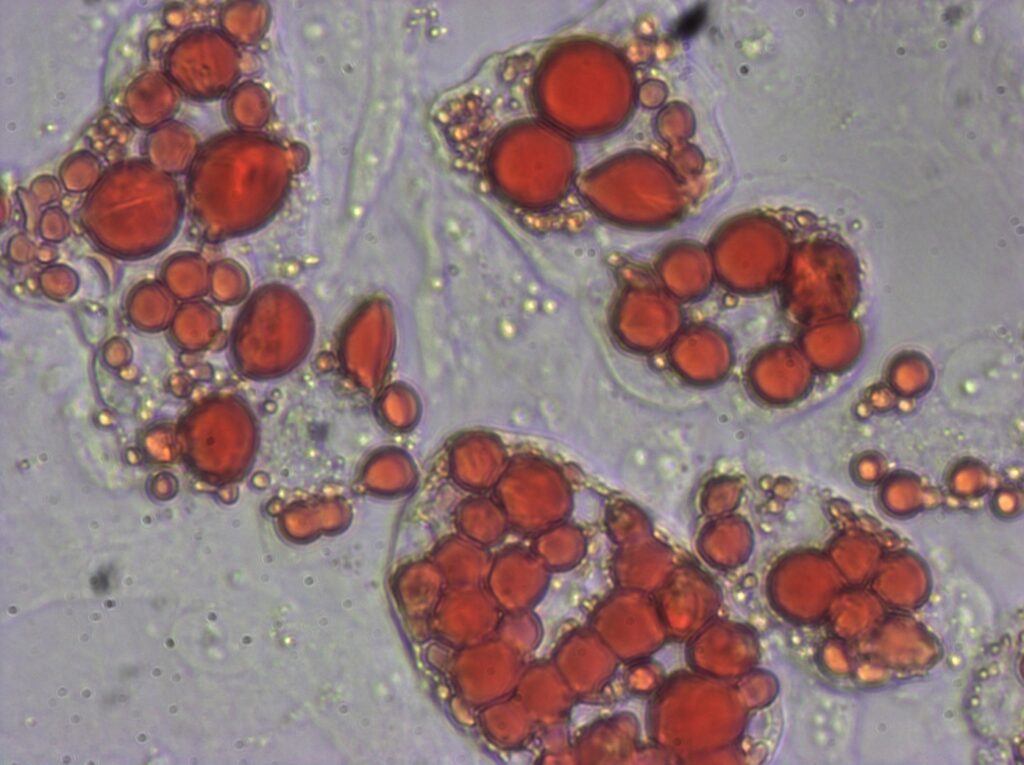
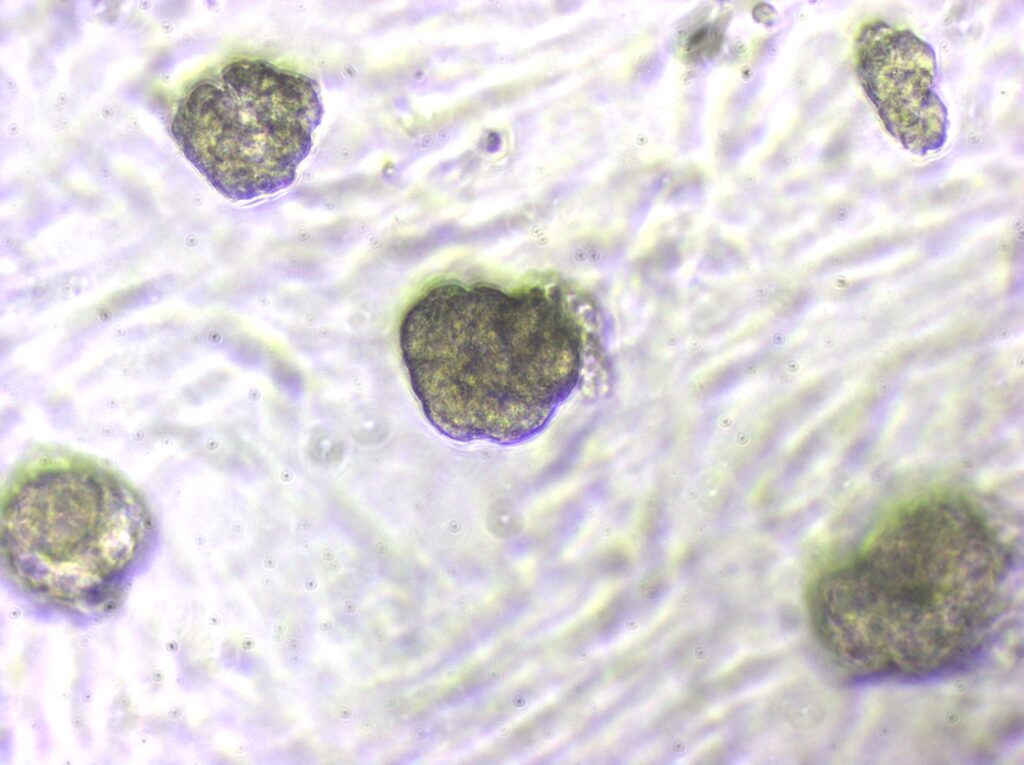
At the heart of our research, we are interested in delineating key links between host cancer risk factors such as hormone exposure, excess adiposity, metabolic factors and their effects on tissue stem/progenitor cells which could serve as seeds in cancer pathogenesis.
Increased adiposity and obesity are global health concerns that increase the risk of several cancers including breast cancer. Since PDGFRα+ PREF-1+ mesenchymal progenitors exhibit a capacity to generate adipocytes and breast epithelial cells, we are studying their dynamics and contribution to epithelial versus adipocyte lineages in an obesogenic microenvironment. Further, breast cancer mouse models in a lineage tracing background are being developed to examine mesenchymal lineage fate during cancer initiation, progression and metastasis. Importantly, we are extending our findings in mouse to human breast tissue, and characterizing mesenchymal stem/progenitor cells and delineating their function in the human breast microenvironment in normal, high-risk and cancer states.
- The dynamics of mesenchymal cells in fallopian tube biology and ovarian cancer:
High grade serous carcinoma is the most common and aggressive subtype of ovarian cancer and its cells of origin have been reported in the ovarian surface epithelium, in the ovarian hilum and in fallopian tube fimbriae. The fallopian tube and ovary have stromal compartments but the role of mesenchymal lineages during epithelial regeneration and cancer formation in these tissues are unknown. We are employing our toolbox of lineage tracing models, stem/progenitor cell assays and organoid systems to decipher the fate and function of mesenchymal progenitors in the ovarian and fallopian tube microenvironment.